- a评估3种皮疹在5个部位的严重程度和皮损面积,总分60分;
- b评估3种皮疹在5个部位的严重程度,总分20分;
- c轻度为仅有轻度皮疹,无论面积大小;中度为严重皮疹,累及皮肤面积<10%;重度为严重皮疹,累及皮肤面积 10% ~ 30%;更重度为严重皮疹,累及皮肤面积>30%;
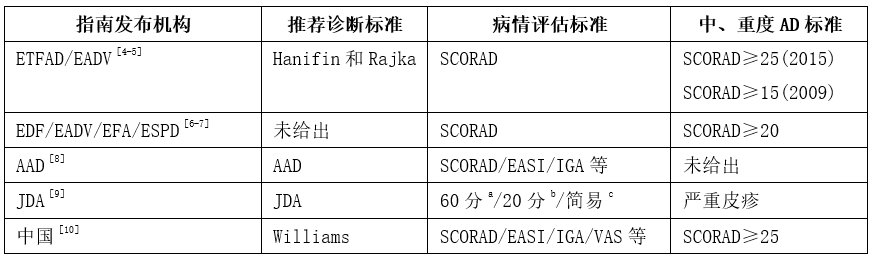
- ETFAD/EADV:欧洲特应性皮炎工作组/欧洲皮肤性病学会湿疹工作组;
- EDF/EADV/EFA/ESPD:欧洲皮肤病论坛、欧洲皮肤性病学会、欧洲过敏协会、欧洲儿童皮肤病学会;
- AAD:美国皮肤病学会;
- JDA:日本皮肤病学会;
- SCORAD/EASI/IGA/VAS:特应性皮炎积分指数/湿疹面积和严重程度指数/研究者总体评分/瘙痒程度视觉模拟尺评分。
在欧洲特应性皮炎工作组/欧洲皮肤性病学会湿疹工作组(ETFAD/ EADV)指南中,2009年SCORAD≥15为中、重度AD,2015年则改为SCORAD≥25为中、重度AD,数据表明了中、重度AD的界定标准较之前更为严格。
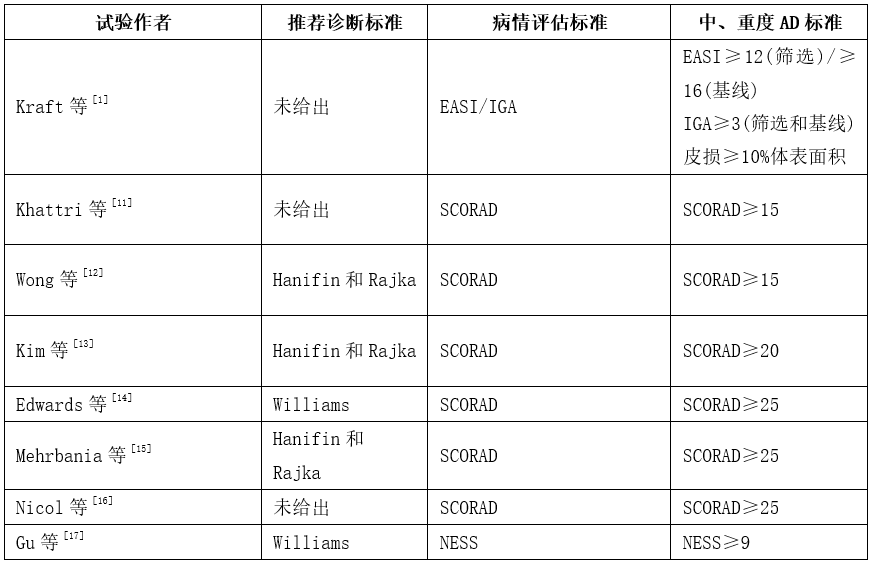
Williams诊断标准简单易行,且特异性和敏感性与Hanifin和Rajka标准相似,更适用于临床实践需要,所以中国推荐采用Williams诊断标准诊断 AD[15]。SCORAD评分标准快速、简单,包括客观表现(皮损面积、皮损严重程度)和主观症状(瘙痒和影响睡眠程度),在世界范围内应用广泛,曾被一些学者认为是评价AD病情严重程度的黄金标准。因此,推荐采用SCORAD评分法评估AD病情严重程度,以SCORAD≥25为界定中、重度AD的标准。


- Kraft M, Worm M. Dupilumab in the treatment of moderate -to -severe atopic dermatitis[J]. Expert Rev Clin Immunol, 2017, 13(4):301-310. DOI:10.1080/1744666X.2017.1292134.
- Hamilton JD, Suárez – Fariñas M, Dhingra N, et al. Dupilumab improves the molecular signature in skin of patients with moderateto-severe atopic dermatitis[J]. J Allergy Clin Immunol,2014,134(6):1293-1300. DOI:10.1016/j.jaci.2014.10.013
- 张建中. 特应性皮炎的诊断标准发展及评价[J]. 中华皮肤科杂志, 2017, 50(1):67-69. DOI:10.3760/cma.j.issn.0412-4030.2017.01.025.
- Wollenberg A, Oranje A, Deleuran M, et al. ETFAD/EADVeczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients[J]. J Eur Acad Dermatol Venereol, 2016, 30(5):729-747. DOI:0.1111/jdv.13599.
- Darsow U, Wollenberg A, Simon D, et al. ETFAD/EADV eczema task force 2009 position paper on diagnosis and treatment of atopic dermatitis[J]. J Eur Acad Dermatol Venereol, 2010, 24(3):317-328. DOI:10.1111/j.1468-3083.2009.03415.x.
- Ring J, Alomar A, Bieber T, et al. Guidelines for treatment of atopic eczema(atopic dermatitis)part I[J]. J Eur Acad Dermatol Venereol, 2012, 26(8):1045-1060. DOI:10.1111/j.1468-3083.2012.04635.x.
- Ring J, Alomar A, Bieber T, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) Part II[J]. J Eur Acad Dermatol Venereol, 2012, 26(9):1176 – 1193. DOI:10.1111/j.1468-3083.2012.04636.x.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis:section 1. Diagnosis and assessment of atopic dermatitis[J]. J Am Acad Dermatol, 2014,70(2):338-351. DOI:10.1016/j.jaad.2013.10.010.
- Saeki H, Nakahara T, Tanaka A, et al. Clinical Practice Guidelines for the Management of Atopic Dermatitis 2016[J]. J Dermatol, 2016, 43(10):1117-1145. DOI:10.1111/1346-8138.13392.
- 中华医学会皮肤性病学分会免疫学组, 等. 中国AD诊疗指南(2020版). 中华皮肤科杂志2020年2月第53卷第2期Chin J Dermatol, February 2020, Vol. 53, No. 2 .
- Khattri S, Brunner PM, Garcet S, et al. Efficacy and safety of ustekinumab treatment in adults with moderate -to – severe atopic dermatitis[J]. Exp Dermatol, 2017, 26(1):28-35. DOI:10.1111/exd.13112.
- Wong SM, Ng TG, Baba R. Efficacy and safety of sodium hypochlorite(bleach)baths in patients with moderate to severe atopic dermatitis in Malaysia[J]. J Dermatol, 2013, 40(11):874-880. DOI:10.1111/1346-8138.12265.
- Kim HS, Lee JH, Roh KH, et al. Clinical Trical of human umbilical cord blood – derived stem cells for the treatment of moderate – to – severe atopic dermatatitis:phase I/IIa studies[J].Stem Cells, 2017, 35(1):248-255. DOI:10.1002/stem.2401.
- Edwards AM, Bibawy D, Matthews S, et al. Long-term use of a 4% sodium cromoglicate cutaneous emulsion in the treatment ofmoderate to severe atopic dermatitis in children[J]. J Dermatolog Treat, 2015, 26(6):541 – 547. DOI:10.3109/09546634.2015. 1034077.
- Mehrbania M, Choopani R, Fekri A, et al. The efficacy of whey dermatitis in adults:a randomized, double – blind, placebo -controlled clinical trial[J]. J Ethnopharmacol, 2015, 172:325 -332. DOI:10.1016/j.jep.2015.07.003.
- Nicol NH, Boguniewicz M, Strand M, et al. Wet wrap therapy in children with moderate to severe atopic dermatitis in a multidisciplinary treatment program[J]. J Allergy Clin Immunol Pract, 2014, 2(4):400-406. DOI:10.1016/j.jaip.2014.04.009.
- Gu SX, Zhang AL, Coyle ME, et al. Chinese herbal medicine granules (PTQX) for children with moderate to severe atopic eczema:study protocol for a randomised controlled trial[J].Trials, 2015, 16:294. DOI:10.1186/s13063-015-0806-y.
有效期:2023年7月27日
本编号仅作为对本文章所涉及的相关药物所属治疗领域科学和临床数据来源真实性的确认,不作为对本文章全部内容准确性、时效性和完整性的确 认和保证;本文章仅供医疗卫生专业人士为学术交流或了解医学资讯目的使用,不构成对任何药物或治疗方案的推荐和推广。本文章所含信息不应代替医疗卫生专业人士提供的医疗建议。



Leave a Reply